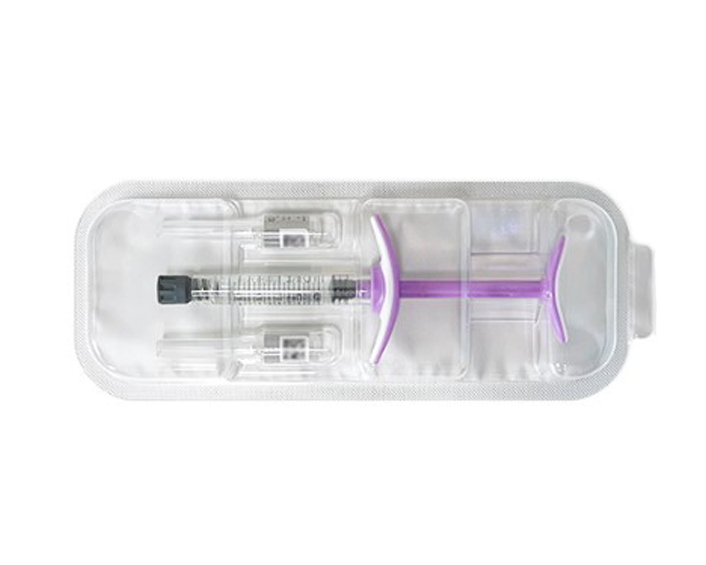
Description
without lot number
[Ingredient]: Cross-linked Hyaluronic Acid 22mg/ml+0.3%lidocaine
[Average particle size]: 900μm
[Indication]: Facial tissue augmentation by injection into areas in which restoration is required. Typically used for treatment of facial wrinkles and folds and also for augmentation of lips.[Shelf-Life]: 24 months
[Recommended micro-cannula]: 25G 40mm or 23G 50mm
[How it is supplied]: Pre-filled syringe 1ml/box with 27GX2 Terumo Thin-wall needles
[ APPLICATION SPOTS ]
[Clinical Trial]
By measuring the mean WSRS of both groups at +26weeks (visit7) after the injection of nasolabial folds, it was demonstrated that the clinical efficacy of YVOIRE volume s was equivalent to that of Product B.